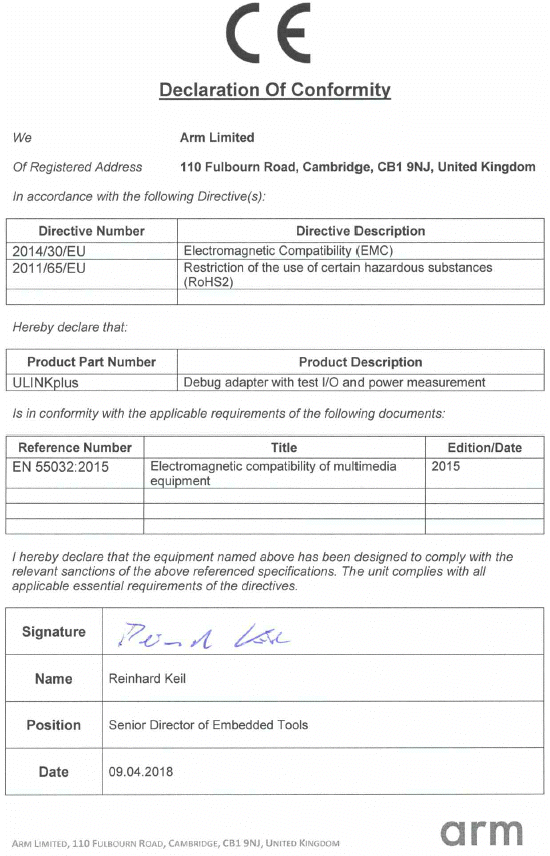
Declaration Of Conformity Template
Declaration Of Conformity Template - Web download free word declaration of conformity templates available for use: By signing the doc you take full responsibility for your product's compliance with the applicable eu law. Web declaration of conformity templates for ukca marking are available to purchase from our shop at product compliance support. Web uk declaration of conformity the uk declaration of conformity is a document which must be drawn up for most products lawfully bearing a ukca marking before they are placed on the market. Do you need an independent assessment? Also sometimes called self declaration of conformity) is one way to show that a product, process or service conforms to a standard or technical regulation, in which a supplier provides written assurance of conformity to the specified requirements.
This is a free template, provided by openregulatory. Web download free word declaration of conformity templates available for use: Web a declaration of conformity (doc) is a document stating that a product, usually electronic, meets the standards to which it must legally adhere, such as safety regulations. Web a declaration of conformity (doc) is mandatory for all products covered by one or more ‘ce marking’ directives. It may include a colour image of sufficient clarity to enable the identification of.
Web download free word declaration of conformity templates available for use: By signing the doc you take full responsibility for your product's compliance with the applicable eu law. Web the ‘eu declaration of conformity’ (which is the most common term used in ce marking) is a document confirming that the product is placed in the market in accordance with the.
Web declaration of conformity templates for ukca marking are available to purchase from our shop at product compliance support. This is a free template, provided by openregulatory. This guidance explains how to use the ukca marking. If you would like to know more about ukca marking or declaration of conformity documents for ukca marking then you will find more information.
For information on placing products on the market, see the guidance on placing products on the market in great britain using uk. Web the eu declaration of conformity (doc) is a legal document, wherein a manufacturer formally declares the compliance of a product with the essential health, safety and environmental requirements of the relevant directives. Complying with fcc standards is.
Web in the united states, the fcc declaration of conformity is a certification process for electronic products that emit radiofrequency (rf) energy. Web you can find the conformity assessment procedures applicable to your medical device class set out in annexes ix to xi of the mdr: Web draft and sign an eu declaration of conformity. Templates for a chemical safety.
In this article you will learn: Web print this page. Web declaration of conformity templates for ukca marking are available to purchase from our shop at product compliance support. Templates for a chemical safety report (csr) annotated templates for an exposure scenario (es) templates for applications for authorisation. By signing the doc you take full responsibility for your product's compliance.
Declaration Of Conformity Template - In this article you will learn: Also sometimes called self declaration of conformity) is one way to show that a product, process or service conforms to a standard or technical regulation, in which a supplier provides written assurance of conformity to the specified requirements. You need to check if your product has to be tested by a. 4.5/5 (7,518 reviews) Web download free word declaration of conformity templates available for use: Web draft and sign an eu declaration of conformity.
Web print this page. Web you can find the conformity assessment procedures applicable to your medical device class set out in annexes ix to xi of the mdr: This article gives some guidance on creating the document and avoiding simple mistakes. Mdd/ivdd, mdr/ivdr & ukca marking. In this guide for importers and amazon sellers, we explain how to create a doc, associated costs, relevant products, and much more.
Web Print This Page.
Web an eu declaration of conformity (doc) is a mandatory document that you as a manufacturer or your authorised representative need to sign to declare that your products comply with the eu requirements. Web in the united states, the fcc declaration of conformity is a certification process for electronic products that emit radiofrequency (rf) energy. You need to check if your product has to be tested by a. Web download free word declaration of conformity templates available for use:
Web You Can Find The Conformity Assessment Procedures Applicable To Your Medical Device Class Set Out In Annexes Ix To Xi Of The Mdr:
For information on placing products on the market, see the guidance on placing products on the market in great britain using uk. How to write a declaration of conformity By signing the doc you take full responsibility for your product's compliance with the applicable eu law. Once your product bears the ce marking — if the competent national authority requests — you must provide them with all the information and supporting documentation concerning ce marking.
Also Sometimes Called Self Declaration Of Conformity) Is One Way To Show That A Product, Process Or Service Conforms To A Standard Or Technical Regulation, In Which A Supplier Provides Written Assurance Of Conformity To The Specified Requirements.
Web the following forms and templates, to be used in the context of reach, can be downloaded from this webpage. Web a declaration of conformity (doc) is mandatory for all products covered by one or more ‘ce marking’ directives. Do you need an independent assessment? Web this declaration of conformity is issued under the sole responsibility of the manufacturer (or installer):
Web Australian Declaration Of Conformity Templates (Medical Devices) Information And Templates For Manufacturers Making An Australian Declaration Of Conformity.
Web declaration of conformity templates for ukca marking are available to purchase from our shop at product compliance support. Web the eu declaration of conformity is an important legal document in which the manufacturer declares the conformity of their medical device. Web if you market your own products, sell on ecommerce platforms such as amazon, export to the eu, write user manuals, or work in r&d, you'll likely need to deal with a declaration of conformity (doc). Once they have done this, they create and sign a document called an eu declaration of conformity (doc) to underwrite that their product satisfies all.