Chemical Makeup Of Aspirin
Chemical Makeup Of Aspirin - Acetic anhydride reacts with water to form two molecules of acetic acid Web the formula for aspirin is (c9h8o4) aspirin is acetylsalicylic acid. This structure is also available as a 2d mol file or as a computed 3d sd file. Web aspirin is an antiplatelet drug that inhibits cyclooxygenase or cox. Web aspirin causes high anion gap metabolic acidosis and respiratory alkalosis. Bob gibbons/science photo library since ancient times, people have been using willow to treat pain and.
By looking at its chemical structure, you'll see that it's composed of three different types of atoms: This structure is also available as a 2d mol file or as a computed 3d sd file. Acetic anhydride reacts with water to form two molecules of acetic acid Web aspirin and salicylic acid are stable compounds and are poorly water soluble at acidic and neutral ph. This includes physicochemical properties of the compounds as well as methods for determination of aspirin and its metabolites.
Web the chemical name of aspirin is acetylsalicylic acid. Specific inflammatory conditions which aspirin is used to treat include kawasaki disease, pericarditis, and rheumatic fever. The 3d structure may be viewed using java or javascript. Web aspirin causes high anion gap metabolic acidosis and respiratory alkalosis. Web the formula for aspirin is (c9h8o4) aspirin is acetylsalicylic acid.
This lab supports the following units, topics and learning objectives: Web aspirin 1 was the molecule of the week for june 4, 2012. This includes physicochemical properties of the compounds as well as methods for determination of aspirin and its metabolites. The physiologic effect of aspirin is by means of decreased prostaglandin production and decreased platelet aggregation. Web these notes.
Web conduct a chemical reaction to produce aspirin. Specific inflammatory conditions which aspirin is used to treat include kawasaki disease, pericarditis, and rheumatic fever. Web aspirin is a white, crystalline, mildly acidic chemical with a melting point of 136 °c (277 °f) and a boiling temperature of 140 °c (284 °f). This structure is also available as a 2d mol.
The 3d structure may be viewed using java or javascript. Acetylsalicylic acid is a very common cause of accidental poisoning in young children. In this lab, students will design an experiment to test the time and completeness of dissolution of various types of aspirin in different ph environments. Web aspirin is a white, crystalline, mildly acidic chemical with a melting.
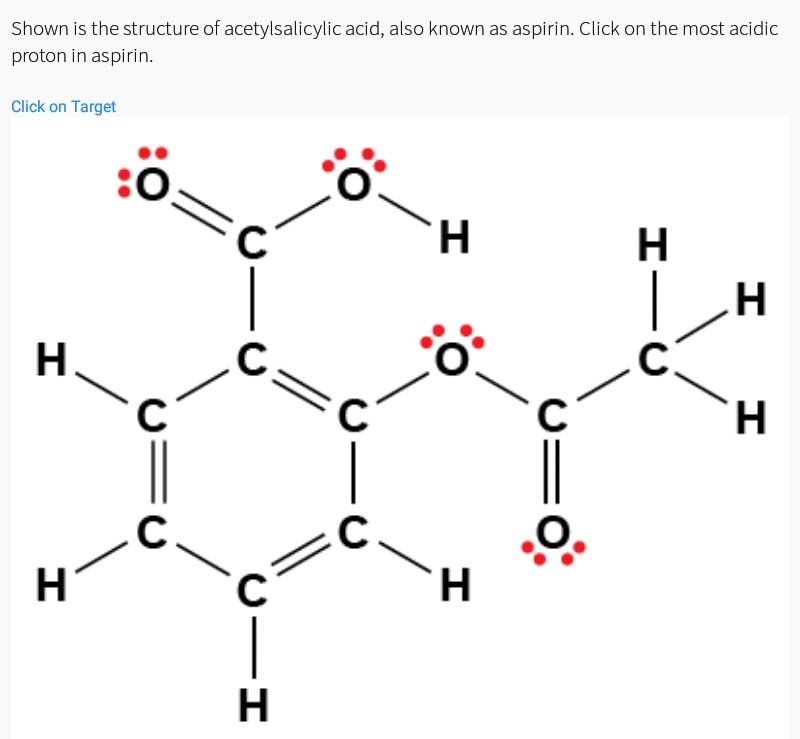
The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. Web synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: This includes physicochemical properties of the compounds as well as methods for determination of aspirin and its metabolites. The mechanism of action of aspirin is as.
Web aspirin causes high anion gap metabolic acidosis and respiratory alkalosis. Analyze the aspirin and estimate its purity. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid, catalyzed by sulfuric acid reaction scheme 2: Web these notes accompanied the 1959 nlm exhibit that detailed the history of aspirin, from the drug’s roots as willow bark used.
It is odourless, colourless to white crystals or crystalline powder. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid, catalyzed by sulfuric acid reaction scheme 2: The acetyl group on aspirin is hydrolzed and then bonded to the alcohol group of serine as an ester. Web aspirin causes high anion gap metabolic acidosis and respiratory alkalosis..
Web aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. This structure is also available as a 2d mol file or as a computed 3d sd file. Web conduct a chemical reaction to produce aspirin. Web these notes accompanied the 1959 nlm exhibit that detailed the history of aspirin, from the drug’s roots as willow.
This lab supports the following units, topics and learning objectives: This structure is also available as a 2d mol file or as a computed 3d sd file. Web conduct a chemical reaction to produce aspirin. Compounds of salicylic acid are found in some plants, notably white willow and meadowsweet ( spirea ulmaria ). The mechanism of action of aspirin is.
The 3d structure may be viewed using java or javascript. Web aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. Aspirin, derivative of salicylic acid that is a mild nonnarcotic analgesic ( pain reliever) useful in the relief of headache and muscle and joint aches. Web the formula for aspirin is (c9h8o4) aspirin is acetylsalicylic.
This lab supports the following units, topics and learning objectives: Web these notes accompanied the 1959 nlm exhibit that detailed the history of aspirin, from the drug’s roots as willow bark used in ancient times, to the distilling of key chemicals to make the aspirin pills that we are familiar with now. The high anion gap comes from the addition.
Chemical Makeup Of Aspirin - Web aspirin is the generic medical name for the chemical acetylsalicylic acid, a derivative of salicylic acid. The molecular weight of aspirin is 180.16g/mol. Web aspirin and salicylic acid are stable compounds and are poorly water soluble at acidic and neutral ph. At 25 °c (77 °f), its acid dissociation constant is 3.5. The molecular formula for acetylsalicylic acid is c 9 h 8 o 4 and the expanded formula is ch 3 cooc 6 h 4 cooh. This includes physicochemical properties of the compounds as well as methods for determination of aspirin and its metabolites. Web willow and myrtle trees contain salicylic acid, a close analog of modern aspirin. Specific inflammatory conditions which aspirin is used to treat include kawasaki disease, pericarditis, and rheumatic fever. Web the formula for aspirin is (c9h8o4) aspirin is acetylsalicylic acid. Web synthesis of aspirin (acetylsalicylic acid) reaction scheme 1:
Web aspirin is the generic medical name for the chemical acetylsalicylic acid, a derivative of salicylic acid. Acetylsalicylic acid is a very common cause of accidental poisoning in young children. By looking at its chemical structure, you'll see that it's composed of three different types of atoms: Web the chemical name of aspirin is acetylsalicylic acid. The acetyl group on aspirin is hydrolzed and then bonded to the alcohol group of serine as an ester.
At 25 °c (77 °f), its acid dissociation constant is 3.5. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid, catalyzed by sulfuric acid reaction scheme 2: Compounds of salicylic acid are found in some plants, notably white willow and meadowsweet ( spirea ulmaria ). The molecular weight of aspirin is 180.16g/mol.
Web willow and myrtle trees contain salicylic acid, a close analog of modern aspirin. This lab supports the following units, topics and learning objectives: It is a venerable pain remedy and anticlotting agent that for decades has been recommended by physicians in low doses (81 mg) as a prophylactic against the risk of heart attack and stroke.
Acetylsalicylic acid is a very common cause of accidental poisoning in young children. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid, catalyzed by sulfuric acid reaction scheme 2: The high anion gap comes from the addition of salicylic acid as well as the generation of lactic acid (due to the uncoupling of oxidative phosphorylation causing.
Specific Inflammatory Conditions Which Aspirin Is Used To Treat Include Kawasaki Disease, Pericarditis, And Rheumatic Fever.
In this lab, students will design an experiment to test the time and completeness of dissolution of various types of aspirin in different ph environments. The mechanism of action of aspirin is as a cyclooxygenase inhibitor. It is a venerable pain remedy and anticlotting agent that for decades has been recommended by physicians in low doses (81 mg) as a prophylactic against the risk of heart attack and stroke. Web aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride.
Analyze The Aspirin And Estimate Its Purity.
Web aspirin 1 was the molecule of the week for june 4, 2012. Aspirin, derivative of salicylic acid that is a mild nonnarcotic analgesic ( pain reliever) useful in the relief of headache and muscle and joint aches. Acetic anhydride reacts with water to form two molecules of acetic acid The high anion gap comes from the addition of salicylic acid as well as the generation of lactic acid (due to the uncoupling of oxidative phosphorylation causing.
Web Conduct A Chemical Reaction To Produce Aspirin.
Salicylic acid is the main component of aspirin. Web willow and myrtle trees contain salicylic acid, a close analog of modern aspirin. This structure is also available as a 2d mol file or as a computed 3d sd file. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid, catalyzed by sulfuric acid reaction scheme 2:
By Looking At Its Chemical Structure, You'll See That It's Composed Of Three Different Types Of Atoms:
The molecular weight of aspirin is 180.16g/mol. The physiologic effect of aspirin is by means of decreased prostaglandin production and decreased platelet aggregation. Acetylsalicylic acid is a very common cause of accidental poisoning in young children. Web the chemical name of aspirin is acetylsalicylic acid.